
Why is chelation best?
Sumalate® has 40% absorption.3,5 This high absorption is due to the chelation. Iron is surrounded by glycine and aspartic acid which are important amino acids in aiding in iron absorption into the enterocytes. Since absorption is increased, mucosal block is limited and hepcidin is less likely to be released and inhibit ferroportin, the channel which moves ferrous iron out of the enterocytes and into the bloodstream. Therefore, it can be said chelated metals increase expression of ferroportin. Because the iron is chelated, it participates less in chemical reactions such as those with absorption inhibitors. Sumalate® can also be absorbed in the proximal or distal duodenum due to its chelation and bioavailability, and therefore is not limited to the proximal duodenum like ferrous salts.
Chelated iron in the form of Sumalate® (ferrous asparto glycinate) is efficiently absorbed, allowing for lower iron dosing1-5
- Highly soluble, about 3x more bioavailable than ferrous sulfate1-5
- Less reactive with biologic tissues, and may be taken with or without food1,2,5,6
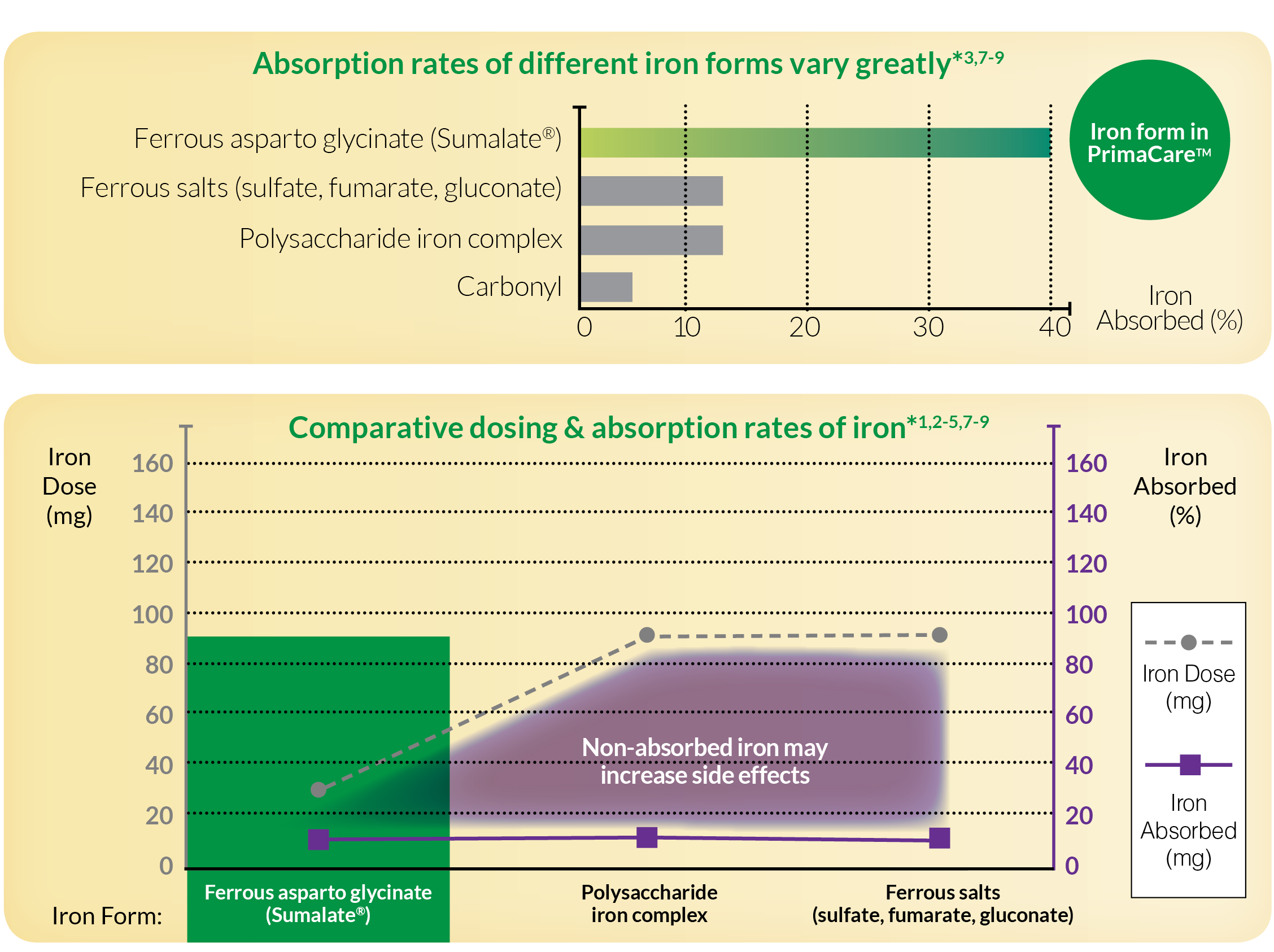
*Bioavailability ranges for irons can vary greatly and may depend on iron form, dose, dose regimen, and other ingredients in the product formulation. Representative bioavailability is dervied from published studies of the iron forms alone.
Pay no more than $20 per script* with our
eVoucherRx™ Savings Program – No Coupon Required!
Sign up for news and future savings
